Finding out how to understand the Electromagnetic Spectrum

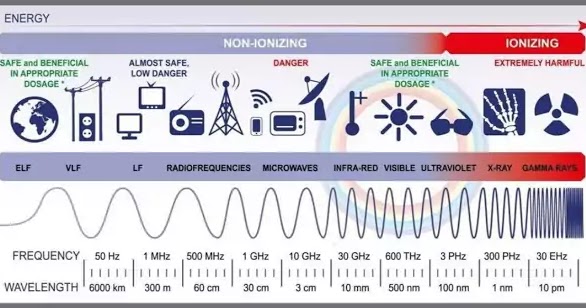
The electromagnetic spectrum describes the range of electromagnetic waves, ranging from visible light to gamma radiation. It is a crucial part of science and understanding the electromagnetic spectrum is essential. In this article I am going to discuss several of the major aspects of this spectrum and the way they work.
Infrared
Infrared refers to the radiation spectrum electromagnetic that goes beyond visible light spectrum. Infrared spectrum is utilized to determine the physical properties that objects exhibit. It is also utilized in night equipment for night vision.
Generally, infrared is classified into near infrared and infrared. Near infrared is the wavelength range that comprises the lowest frequencies. These wavelengths are within the range of one to five microns. There are two long and intermediate infrared bands. Each is characterized by the unique wavelengths.
The most well-known use for infrared is found in military night vision goggles. These glasses convert infrared light into the visible wavelengths for night-time viewing. Infrared light is also used for wired and wireless communication.
There is no evidence of a link between infrared radiation and skin cancer. However, em light spectrum is known that the International Commission on Non-Ionizing Radiation Protection (ICNIRP) has issued guidance on the exposure limits to infrared and visible radiation that is incoherent.
Visible light
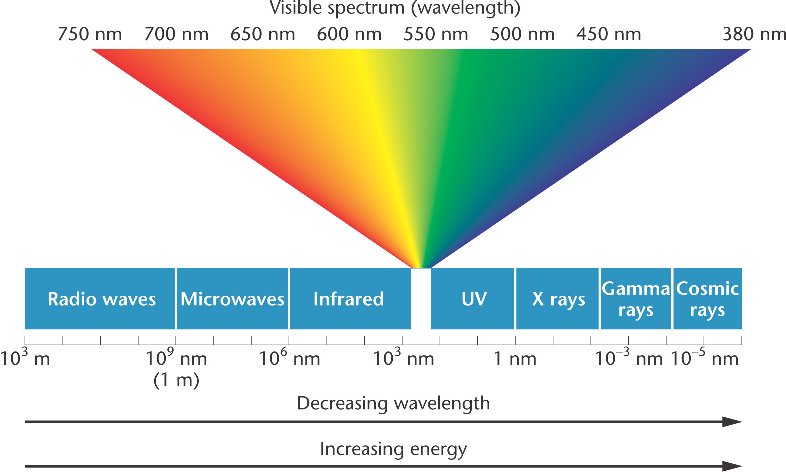
Visible light is part of the electromagnetic spectrum. The Sun is our main lighting source. Other sources of visible light are the moon and stars. It is essential to realize that we are unable to see ultraviolet and infrared wavelengths. But, we can see the blue and red light. The two colours blend creating what we call white light.
There are also many more obscure components to the electromagnetic spectrum, such as radio waves and infrared. Some of these are used for television, radio as well as mobile communication. But, the best way to utilize these is to create the right kind of filter. This way we can limit the harmful effects of these elements on our body. Additionally, we can build a virtual environment where we can safely examine these elements, even with our eyes off.
Although the longest and shortest wavelengths of visible light might be most noticeable however, the most efficient and pleasing to the eye can be found in the infrared shortwave (SWIR) and microwave frequencies.
UV
Ultraviolet (UV) radiation is a part of the electromagnetic spectrum. It can be utilized to fulfill a variety of functions. However, it could also be damaging. UVB and UVC radiations are harmful for eyesight and can lead to skin cancer.
This type of energy can be absorbed by atoms and start chemical reactions. The molecule that is absorbing it will emit visible light or fluoresce.
The spectrum of ultraviolet light is divided into three major categories: the extreme, the near in addition to the further. Typical ultraviolet sources include arc lamps, lasers, and light emitting diodes.
While the wavelengths of UV Rays are smaller than those of X-rays they possess more energy. This is useful for breaking the bonds between chemical compounds. The waves are also known as nonionizing radiation.
In biochemistry the ultraviolet spectrum is commonly utilized to measure the absorption rate of a particular substance. There are numerous types of compounds that exhibit significant absorption bands of light that are visible in UV.
Ultraviolet light forms a part of the spectrum known as electromagnetic, which is produced by the sun. Its range is between 10 and 400 nanometers. Its frequencies are from 800 THz to 30 PHz. However, most people cannot be able to see it.
X-rays
The X-rays, also known as electromagnetic radiation, have high energy. In contrast to gamma rays and UV light, X-rays are smaller than visible light and can penetrate thin objects. They are employed in a range types of applications in medicine, such as imaging bone and tissue. Several types of X-rays exist.
Hard X-rays can be produced when an electron that is incoming collides with an atom. This results in a vacancy within the electron shell of the atom. Another electron could fill in the gap. Or, the electron that is incoming could release an atom. In this case, some of the energy generated by the photon is transferred to the scattering electron.
A X-ray should not be mistaken for the X band, which is a low-energy spectrum that is part of the electromagnetic spectrum. While the two bands overlap by a few hundred nanometers, they do not have the same characteristics.
Since X-rays penetrate the body, they can be utilized in many different ways. For example, X-rays are employed in security screening procedures to detect cracks in baggage. Additionally, they are used in radiotherapy for cancer patients. The X-rays can also be used to identify the structural elements of various materials like cement.
Gamma rays
Gamma Rays are very high-energy types in electromagnetic radiation. In fact, all extremely high energy photons are radiations. These photons are produced by nuclear decay and high-energy Physics experiments. They are among the most energetic photons in the electromagnetic spectrum.
Due to their powerful energy, gamma rays can be capable of reaching deeply into the materials. It is possible for a single gamma ray to penetrate as much as a few feet of lead.
Several high-energy physics experiments produce the gamma radiation. For example the particle beam from a relativistic source directed on by a magnetic field from the hypernova is visible at the distance of 10 billion light years.
Some gamma rays are emitted by the nucleus of some radionuclides after they have gone through the process of radioactive decay. Other sources of gamma rays are atomic transitions, annihilation, and sub-atomic particle interactions.
The majority of gamma rays in astronomy come from different mechanisms. Gamma rays from supernovae and nuclear fallout are among the most energetic forms of electromagnetic radiation. This makes them a great source for exploring the universe.
Certain gamma radiations could cause harm to cells within the body. However, gamma rays are not as ionizing like beta and alpha rays, and therefore are less likely to cause cancer. Nevertheless, gamma rays can alter the DNA structure and may cause burns. Even the smallest amounts of gamma radiations could cause an ionization of the body.
